Sparta Biomedical, a Morrisville, NC-based medical technology company, raised an undisclosed amount in convertible funding.
The round saw participation from angel investors, along with participation from previous backers.
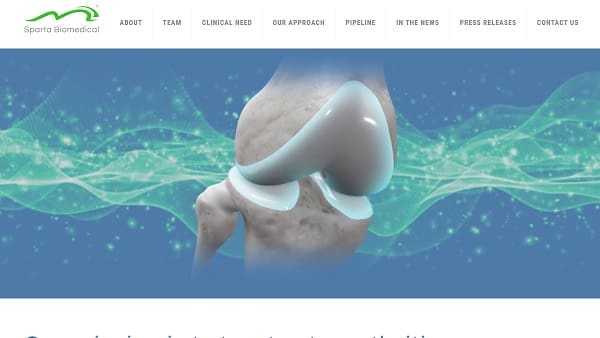
The secured capital will accelerate the company’s preparations for the Investigational Device Exemption study preparations of its breakthrough device, Ormi.
Led by President Dimitrios Angelis, CTO Ben Wiley, and VP and Engineering Head Demetri Siachames, Sparta is a biotech company committed to improving motion, reducing pain, increasing the quality of life of patients and mimics the properties of healthy human cartilage right from day one, providing an innovative solution to this widespread problem. Ormi aims to to treat knee osteoarthritis. It is not approved by the FDA and not commercially available. It is in development. Approvals and clearances are subject to testing, results, and the FDA review process.





